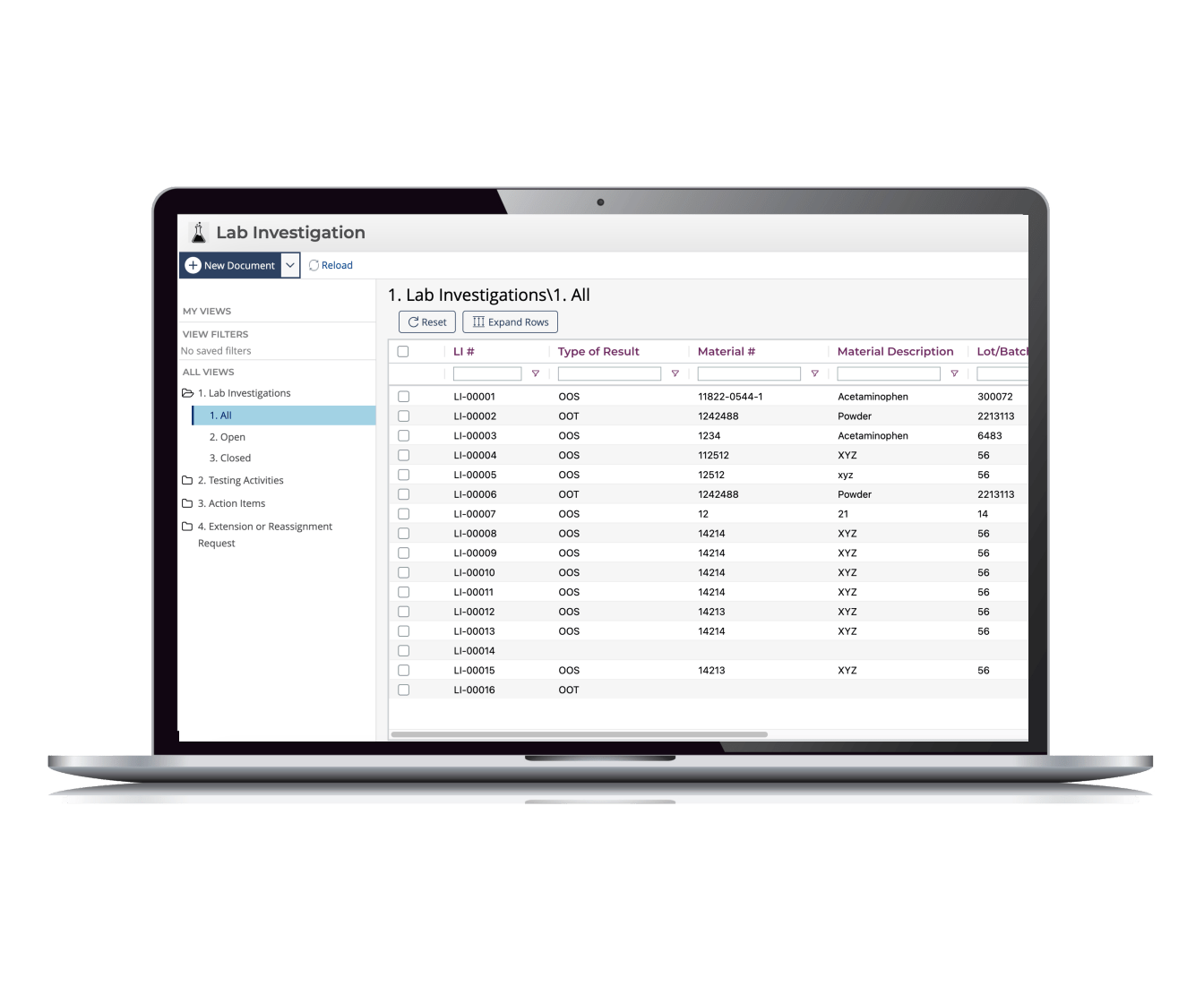
OOS Software APP
A systematic, thorough approach to lab investigations
ETQ Reliance’s® OOS software app helps to document the investigation process of an Out of Specification (OOS) or Out of Trend (OOT) lab test result.
Users now have the ability to investigate an Out of Specification or Out of Trend result to determine the cause of the result, and through the investigation process, conduct re-sampling/re-testing to confirm the test results.